Metalloproteins Vibrational biospectroscopy
Vibrational biospectroscopy and its application to metalloproteins and their mineral catalytic precursors”
- Date 12, 13,&14March 2025 (including theoretical lectures and practical/laboratory sessions)
- Duration 3 days – 15 hours Doctoral School equivalent
- Language English
- Name of the teachers:
- Professor Piotr J. Mak (Department of Chemistry, Saint Louis University, Saint Louis, MO, USA; AMU Invited Professor 2025); Dr Anabella Ivancich (CNRS, Laboratoire de Bioénergétique et Ingénierie des Protéines (BIP)/UMR 7281, CNRS & AMU, Marseille, France); Dr Daniel Ferry (Centre Interdisciplinaire de nanoscience CINAM, Marseille, France).
- Administrative head Eric Pilet
- Number of students Group of 4-8 students
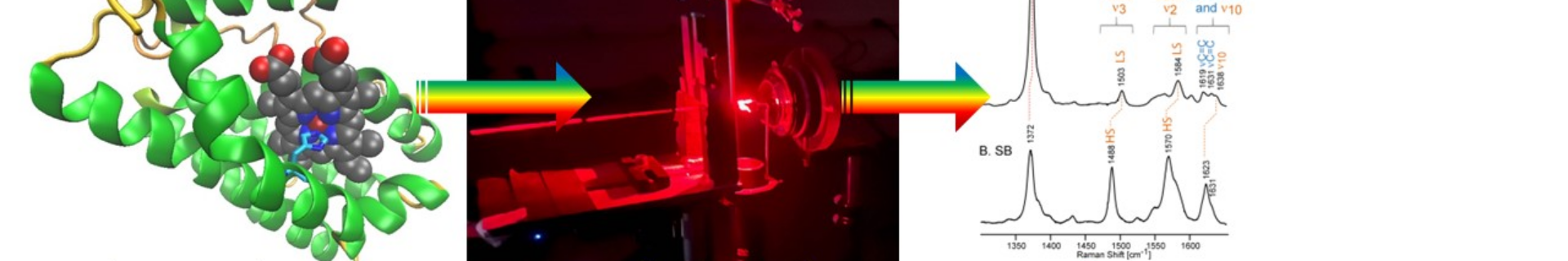
– Course content: The aim is to introduce students to the theory and applications of molecular spectroscopy in biomolecular research. Particular emphasis will be placed on vibrational spectroscopy, especially resonance Raman spectroscopy, to study the structure-function relationship in heme proteins that play essential physiological functions in eukaryotes and pathogens. The course will consist of theoretical lectures and demonstrations/hands-on laboratory experiences. The theoretical part will cover theory and real-life, advanced experimental techniques of vibrational spectroscopy, such as Raman, resonance Raman, and FT-IR, followed by an overview of its application in structural studies of heme iron proteins as well as the non-heme iron precursors (fougerite double-layered minerals). The laboratory sessions will include UV-Vis electronic absorption, Raman and Electron Paramagnetic Resonance (EPR) spectroscopies, highlighting the advantageous output of such complementary techniques in assessing structural and functional aspects of metal-containing systems.
Course Outcomes: Through the completion of the course, students will acquire the following knowledge and skills:
1) Understand the quantum mechanical foundations and symmetry aspects of molecular spectroscopy employed in biochemistry and biophysics.
2) Capability to make an informed choice of a spectroscopic method for structural protein characterization.
3) Practical skills to correctly interpret and extract structural information from vibrational and EPR spectra of heme proteins and iron minerals (green rust).
4) Understanding of the physical factors limiting the applicability of different spectroscopic methods in biomolecular research.
– Students prerequisites.
Basic knowledge of Chemistry/Bioinorganic Chemistry, Biochemistry.
The prior reading of references 1, 2 and 3 will be beneficial to get a better understanding of the theoretical lectures and practical aspects of resonance Raman spectroscopy. Similarly, reference 4, 5 and 6 will facilitate understanding of the experimental sessions (Raman and EPR spectroscopy), given the relatively limited time available.
Prior Reading Assignments:
1) “Resonance Raman Spectra of Heme Proteins and Model Compounds”, Kincaid, J. R. in The Porphyrin Handbook Kadish, K. M.; Smith, K. M.; Guilard, R.; (Eds.) Academic Press, 2000, 7, 225-291.
2) Snyder, N. S.; Mak, P. J. “Structure-function characterization of the mono- and diheme forms of MhuD, a noncanonical heme oxygenase from Mycobacterium tuberculosis”, J. Biol. Chem., 2022. 298, 101475-101790. DOI: 10.1016/j.jbc.2021.101475
3) Mak, P. J.; Gregory, M. C.; Denisov, I. G.; Sligar, S. G.; Kincaid, J. R. “Unveiling the crucial intermediates in androgen production”, Proc. Natl. Acad. Sci. USA 2015, 112, 15856-15861. DOI : 10.1073/pnas.1519376113
References related to the complementary approach of RR ad EPR spectroscopies on heme enzyme:
4) Celis, A.I, Geeraerts, Z., Ngmenterebo, D., Machovina, M.M., Kurker, R.C., Rajakumar, K., Ivancich, A., Rodgers, K.R. Lukat-Rodgers, G.S., DuBois, J.L., Biochemistry 2015, 54, 434–446. DOI: 10.1021/bi501184c
References related to the non-heme iron minerals and Raman spectroscopy.
5) Legrand, L., Sagon, G., Lecomte, S., Chausse, A., Messina, R. Corrosion Science 2001, 43, 1739-1749. https://doi.org/10.1016/S0010-938X(00)00172-4.
6) Farr, O., Gaudu, N., Danger, G., Russell, M.J., Ferry, D., Nitschke, W., Duval, S. J. R. Soc. Interface 2023, 20, 1-9. DOI: 10.1098/rsif.2023.0386
– Location: IMM/UMR 7281 (BIP). Bat. B. (room B201 for lectures and lab B110) Campus Joseph Aiguier, CNRS.
Trainer(s)
Wednesday 12th, March 2025 09:00
Campus J. Aiguier





